What is Ocean Acidification?
INTRODUCTION
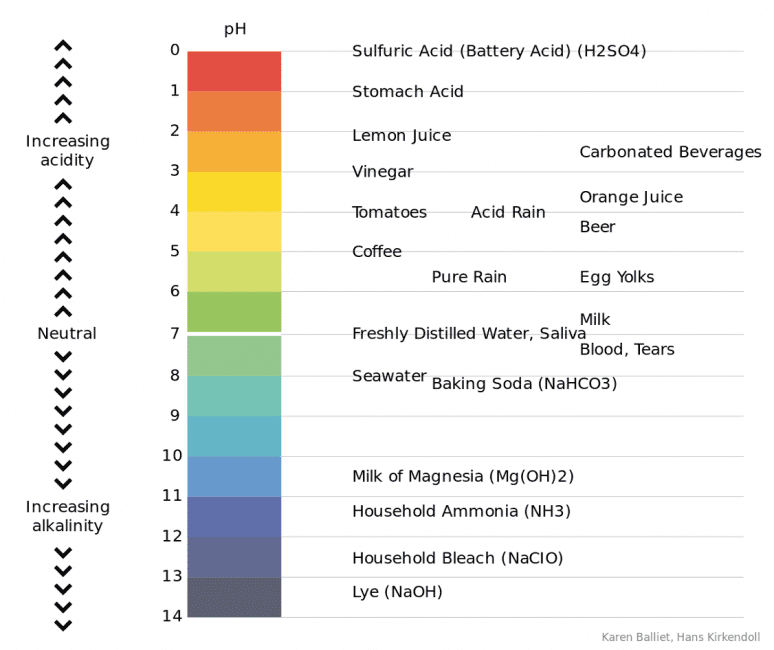
Ocean acidification is a chemical process that makes seawater more acidic. Acidity is measured on the pH scale from 0 to 14, with 7 being neutral. Lower numbers represent a higher acidity and vice versa.
The ocean is naturally slightly alkaline (the opposite of acidic). Before the Industrial Age, the pH of the ocean was around 8.29.
What causes ocean acidification?
INTRODUCTION
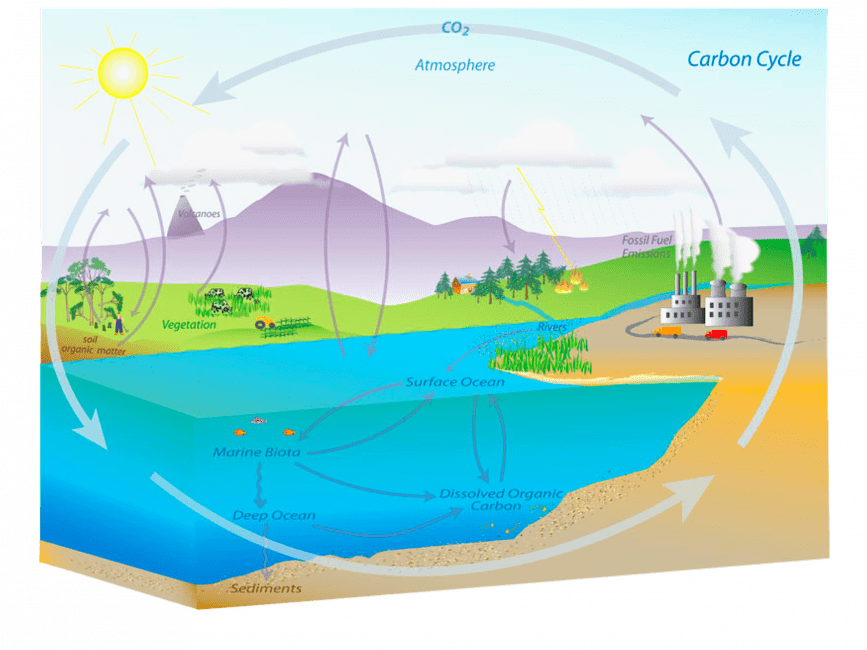
Ocean acidification occurs when the ocean absorbs excess carbon dioxide from the atmosphere. While it is normal for the ocean to absorb some carbon dioxide, humans have released huge amounts of this greenhouse gas very quickly. Since the Industrial Revolution the amount of carbon dioxide in the atmosphere has almost doubled. It builds up in the atmosphere and the ocean is working overtime absorbing the excess.
INTRODUCTION
How does ocean acidification happen?
When carbon dioxide is dissolved in seawater, the ocean (H2O) absorbs carbon dioxide (CO2), carbon dioxide dissolves and is converted into carbonic acid (H2CO3), which makes the ocean more acidic.
Photo credit: Sea Ice by J. Data Imagery on Foter
The ocean is becoming more acidic
WHAT’S CHANGED?
Before the Industrial Age, ocean pH was around 8.29. Since then it has dropped to 8.1. While this change might sound small, it makes a big difference. This is because the pH scale is logarithmic, so a change from 8 pH to 7 pH means a tenfold increase in acidity.
This process is speeding up
In the 13 years between 1994 and 2007, the ocean absorbed four times more carbon dioxide than it did in the almost 200 years between 1800 and 1994.
WHY IT’S IMPORTANT
Impacts of ocean acidification
The current increase in acidity is the fastest known change in ocean chemistry in the past 50 million years, a rate of change that makes it difficult for marine ecosystems to adapt. This rapid transition to a more acidic ocean threatens to destabilize the entire Antarctic marine ecosystem.

Shells dissolving
One of the problems with a more acidic ocean is that it eats away at a mineral in the ocean called calcium carbonate.

Corals threatened
As sea water becomes more acidic it becomes more difficult for cold-water corals to build their skeletons.

Trace metals changing
Trace metals are essential for marine life to survive, and are present in tiny concentrations across the ocean.

Carbon sink
Like plants on land, ocean plants such as kelp, microalgae and other forms of phytoplankton consume carbon dioxide through photosynthesis to create energy for life.
Polar oceans are worst affected
IMPACTS OF OCEAN ACIDIFICATION
Polar oceans are particularly vulnerable to ocean acidification. Around half of the carbon dioxide taken up by the whole ocean is absorbed by the Southern Ocean, as more gas dissolves in cold water than warm water.
In addition, calcium carbonate is naturally low in polar oceans compared to the rest of the world. Species in the polar regions have adapted to the limited supply, but ocean acidification makes calcium carbonate even less available, making an already scarce resource even more hard to find.

WE ARE ENTERING UNCHARTED TERRITORY
Into the unknown
According to researchers, if we continue with ‘business as usual’ carbon dioxide emissions, by 2300 the ocean will be more acidic than it has been in around 20 million years, and the rate of change could be faster than any time in history.
We are entering uncharted territory, racing towards an ocean with a chemical composition we have never seen before.
KEEP LEARNING
Related reading
Scientific consultation: Bea Pena-Molino, physical oceanographer in the Climate Science Centre (Ocean & Atmosphere) at the Commonwealth Scientific and Industrial Research Organisation (CSIRO).
Now that you’ve learned about how carbon dioxide is making the Southern Ocean more acidic, read on to learn more about extraordinary Antarctica.
 ASOC
ASOC







